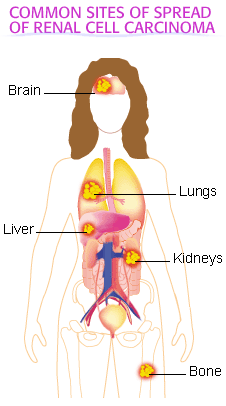
Pulmonary metastases of renal cancer
Data in short:
5-year overall survival:
0% without local IL-2
21% with local IL-2
Local IL-2 seems to be the best treatment
NOTE
In contrast to other regimes of local IL-2 therapy, the application in this disease involves a high dose of IL-2 (and relative high costs) by continued daily application of 1 ampule per day (300 euros per treament).
Therefore this research is funded independently from other studies by the group of Prof. E. Huland.
5-year overall survival:
0% without local IL-2
21% with local IL-2
Local IL-2 seems to be the best treatment
NOTE
In contrast to other regimes of local IL-2 therapy, the application in this disease involves a high dose of IL-2 (and relative high costs) by continued daily application of 1 ampule per day (300 euros per treament).
Therefore this research is funded independently from other studies by the group of Prof. E. Huland.
References
Huland et al. (1994) J. Cancer Res. Clin. Oncol. 120:221
Huland et al. (1997) Cancer J. Sci. Am..3 Suppl.1 , S68
Huland & Heinzer (2003) Curr. Op. Urol. 13, 451
Huland et al. (2003) Folia Biol (Praha) 49,183
Huland & Heinzer (2004) Curr. Op. Urol. 14, 239
Huland et al. (2004) Urologe A online
Huland et al. (1997) Cancer J. Sci. Am..3 Suppl.1 , S68
Huland & Heinzer (2003) Curr. Op. Urol. 13, 451
Huland et al. (2003) Folia Biol (Praha) 49,183
Huland & Heinzer (2004) Curr. Op. Urol. 14, 239
Huland et al. (2004) Urologe A online
Publications
Huland E, Heinzer H, Huland H. (1994)
Inhaled interleukin-2 in combination with low-dose systemic interleukin-2 and interferon alpha in patients with pulmonary metastatic renal-cell carcinoma: effectiveness and toxicity of mainly local treatment.
J Cancer Res Clin Oncol.120(4):221-8.
We describe here a mainly topical interleukin-2 (IL-2) application in pulmonary metastatic renal-cell carcinoma: a high-dose long-term inhalation of IL-2 (90% of IL-2 dose) and low-dose systemic subcutaneous IL-2 (10% of IL-2 dose) and systemic subcutaneous interferon alpha (IFN alpha). The effectiveness of this treatment is remarkable. No pulmonary metastases progressed during treatment. One complete response, 8 partial responses, and 6 cases of stable disease were achieved in the lungs of the 15 patients. In addition, 3 of 7 patients had partial responses and 1 of 7 had stabilization of non-pulmonary metastases. Overall response according to WHO criteria was 1 complete response, 6 partial responses, 2 mixed responses, 5 stable diseases and 1 progressive disease. Toxicity was low. Only WHO grade I toxicity occurred, except for a single grade II event (bronchospasm). This allowed long-term ambulatory treatment (1-23 months) inclusion of high-risk patients, and inclusion of patients with advanced disease. The expected mean survival of patients was 9.9 months, the actual mean survival is now 19.1 months, and 11 of 15 patients are still alive. Quality of life during treatment was good. Inhalation of IL-2 serves as a clinical model for high effectiveness and low toxicity of long-term local IL-2 application. We conclude that mainly local treatment might be the key to successful nontoxic use of IL-2 in cancer patients.
Publication Types: Clinical Trial
Huland E, Heinzer H, Mir TS, Huland H. (1997)
Inhaled interleukin-2 therapy in pulmonary metastatic renal cell carcinoma: six years of experience.
Cancer J Sci Am. 3 Suppl 1:S98-105.
PURPOSE: Patients with advanced metastatic renal cell carcinoma often cannot or do not want to tolerate high-dose systemic interleukin-2 (IL-2) therapy and the toxicity associated with it. To reduce toxicity and still maintain or even increase effectiveness, we developed a method to deliver IL-2 locally for the treatment of pulmonary and mediastinal metastases in metastatic renal cell carcinoma patients. PATIENTS AND METHODS: We report here 6 years of experience treating 116 metastatic renal cell carcinoma patients who had pulmonary or mediastinal metastases with inhaled IL-2. We have utilized three different IL-2 preparations (natural human IL-2 purified from the supernatants of mitogen-activated peripheral blood lymphocytes, glycosylated recombinant IL-2 produced by Chinese hamster ovary cells, and non-glycosylated recombinant IL-2 produced by bacteria). All protocols used high-dose inhalation of IL-2, either exclusively (11%), with coadministration of low-dose systemic IL-2 (33%), or with coadministration of low-dose systemic IL-2 and interferon-alpha (56%). RESULTS: Maximal toxicity per total treatment time was mild (median treatment time, 7.2 months); there was a low incidence (16%) of World Health Organization grade 3 toxicity. Toxicity associated with exclusive inhalation of IL-2 was local and consisted mainly of cough. Thus, patients who could not tolerate high-dose systemic IL-2 were able to tolerate inhalation IL-2 therapy. Progressive pulmonary metastases responded in 15% of patients for a median of 15.5 months (range, 4.1-33 months) and were stabilized in 55% of patients for a median of 6.6 months (range, 3-51.7 months). The overall response rate was 16%; disease was stabilized in 49% of patients and disease progressed in 35% of patients. The overall median response duration was 9.6 months. Median survival was 11.8 months (range, 1.7-68.8 months); expected survival according to risk analysis was 5.3 months. CONCLUSIONS: Inhalation of IL-2 is a nontoxic and effective treatment for patients with progressive pulmonary and mediastinal metastases. Inhaled IL-2 effectively prevented progress of pulmonary metastases in 70% of patients. Furthermore, patients could be treated as outpatients and remain employed. Local administration of IL-2 increases therapeutic effectiveness with little or no toxicity.
Publication Types: Clinical Trial, Controlled Clinical Trial
Huland E, Heinzer H. (2003)
Renal cell carcinoma: novel treatments for advanced disease.
Curr Opin Urol. 13(6):451-6.
PURPOSE OF REVIEW: IL-2 or IFN-alpha induce remissions and prolong life in patients carefully selected for a possibly toxic treatment. However, there is a need for better-tolerated and more effective therapies, especially in patients with co-morbidities and those resistant to systemic immunotherapy. Recent achievements in the treatment of advanced renal cell carcinoma highlight potentially significant improvements. RECENT FINDINGS: Cytoreductive surgery or radiation of metastases seems beneficial in well-selected patients, especially as immunotherapy is available. Immune cells within the tumour correlate with response and survival, indicating the importance of local immune modulation. Such modulation has allowed the introduction of well-tolerated treatments such as the inhalation of IL-2 to control lung metastases, which results in a significant survival benefit for high-risk patients, as suggested by a recent outcome study in 200 patients. Antibody-based tumour targeting against cG250, specifically expressed on renal cell carcinoma, seems to stabilize progressive metastatic disease and does not induce toxicity. Vaccination strategies are also well tolerated, but have not yet shown convincing results in advanced disease. Other approaches have not fulfilled expectations. Thalidomide has significant neurotoxicity and its efficacy was not confirmed in recent studies. Stem cell transplantation has significant toxicity, and cannot yet be recommended, but may have future potential. SUMMARY: Cytokine-based immunotherapy can now be considered standard in the treatment of metastatic renal cell carcinoma. There is good evidence that additional local procedures such as surgery, radiation or the inhalation of IL-2 improve response and survival in metastatic disease with moderate toxicity, resulting in a significant improvement for patients suitable for these approaches.
Huland E, Burger A, Fleischer J, Fornara P, Hatzmann E, Heidenreich A, Heinzer H, Heynemann H, Hoffmann L, Hofmann R, Huland H, Kampfer I, Kindler M, Kirchner H, Mehlhorn G, Moniak TH, Rebmann U, Roigas J, Schneider TH, Schnorr D, Schmitz HJ, Wenisch R, Varga Z, Vinke J. (2003)
Efficacy and safety of inhaled recombinant interleukin-2 in high-risk renal cell cancer patients compared with systemic interleukin-2: an outcome study.
Folia Biol (Praha).49(5):183-90.
Systemic IL-2 is an effective treatment for low to intermediate risk mRCC patients, its efficacy is marginal in high-risk cases. Therefore, other treatment approaches are required for this population. Ninety-four high-risk patients with RCC and pulmonary metastases were treated with inhaled plus concomitant low-dose subcutaneous rhIL-2. Clinical response, survival and safety were compared with those from IL-2 given systemically at the registered dose and schedule in 103 comparable historical controls. In the rhIL-2 INH group, treatment consisted of 6.5 MIU rhIL-2 nebulized 5x/day and 3.3 MIU rhIL-2 SC once daily. The rhIL-2 SYS group received treatment which consisted of intravenous infusion of 18.0 MIU/m2/day rhIL-2 or SC injection of 3.6-18.0 MIU rhIL-2. Some patients in both groups also received IFNalpha. Mean treatment durations were 43 weeks rhIL-2 INH and 15 weeks rhIL-2 SYS. Significantly longer overall survival and progression-free survival durations were observed in the rhIL-2 INH group. The probability of survival at 5 years was 21% for the rhIL-2 INH group. No patients survived 5 years in the rhIL-2 SYS group. A multivariate analysis of overall survival adjusting for differences in baseline characteristics between the two treatment groups resulted in a risk ratio of 0.43 (95% CI 0.30-0.63; P < 0.0001). The data suggested an association between the response (SD or better) and survival, especially in the rhIL-2 INH group. The inhalation regimen was well tolerated. This outcome study suggests that administration of rhIL-2 by inhalation is efficacious and safe in high-risk mRCC patients with pulmonary metastases, who have no other treatment option available.
Publication Types: Clinical Trial
Huland E, Heinzer H. (2004)
Renal cell carcinoma - innovative medical treatments.
Curr Opin Urol. 14:239-244.
PURPOSE OF REVIEW: This review highlights recent innovations of medical treatments in advanced renal cell carcinoma. Because of significant toxicity many patients do not benefit from standard systemic cytokine therapy using interleukin-2, interferon-alpha, or both. Their median progression-free survival is about 2 months and their overall survival is poor. Here we report improvements in patient outcome, focusing on overall survival, progression-free survival and quality of life to meet their needs. RECENT FINDINGS: Rating treatment results requires analysis of patient selection and risk stratification. In well-selected patients systemic interleukin-2 therapy as well as combinations of cytokines (interleukin-2 and interferon-alpha) improve outcome, induce long-term survival and, in a minority of patients, even produce long-lasting complete remissions. Within the group of patients who are not candidates for systemic cytokines, interleukin-2 for pulmonary metastases given locally by inhalation achieves long-term progression-free survival and long-term overall survival similar to high-dose systemic interleukin-2 but without the toxicity associated with it. In patients at high risk for metastatic disease progression-free survival is prolonged by autologous tumor vaccine as reported in a recent randomized study. However, in established metastatic disease no clear benefit has been demonstrated by any of the many recent vaccine protocols. Chemotherapeutic agents and thalidomide generally fail to improve patient outcome but produce significant and sometimes irreversible toxicity. SUMMARY: Cytokine-based immunotherapy can be considered standard in the treatment of metastatic renal cell carcinoma today. Long-term survival and sometimes even cure seems possible in patients using systemic and local (inhaled) applications of interleukin-2 alone or in combination with interferon-alpha. Noncytokine treatment approaches are scientifically interesting but still disappointing from a patient's perspective.
Huland E, Heinzer H, Jorres RA, Loppow D, Huland H. (2004)
Therapeutic approaches in metastatic renal cell carcinoma: local immunotherapy.
Urologe A. (Online First)
Since 1990, aerosol interleukin (IL)-2 has been used to treat pulmonary metastatic renal cell carcinoma (pmRCC). Inhalation therapy deposits a drug into the airways to achieve a high, local, clinical effect while avoiding serious systemic side effects. We report three studies to describe safety and efficacy of aerosol IL-2 in patients with pmRCC. In a multicenter study, 24 patients received exclusive inhalation (study I) of natural IL-2 (three dose levels, 48 weeks) and response and toxicity were evaluated. The survival of high-risk patients (study II) with mainly inhaled IL-2 ( n=94) was compared with that of patients receiving systemic IL-2 ( n=103). In ten patients we analyzed in detail lung function and markers of airway inflammation before and during inhalational IL-2 therapy (study III). Study I: The response of exclusive inhalation was 33.3% at 3 months and 16.7% at 6 months. Treatment was well tolerated, cough being the most frequent adverse event. Study II: The probabilities of survival at 5 years were 21% for the inhalational group and 0% for the systemic group. Study III: Inhaled IL-2 induced a moderate decline of forced expiratory volume (FEV), while exhaled nitric oxide (NO) and sputum eosinophils rose accompanied by moderate cough and dyspnea. In conclusion, inhalational IL-2 combines good efficacy and improves tolerability. This is especially important for patients who are not able to benefit from systemic IL-2 therapy. Whether the local eosinophilic response additionally supports the antitumor effect remains a challenging question.
Inhaled interleukin-2 in combination with low-dose systemic interleukin-2 and interferon alpha in patients with pulmonary metastatic renal-cell carcinoma: effectiveness and toxicity of mainly local treatment.
J Cancer Res Clin Oncol.120(4):221-8.
We describe here a mainly topical interleukin-2 (IL-2) application in pulmonary metastatic renal-cell carcinoma: a high-dose long-term inhalation of IL-2 (90% of IL-2 dose) and low-dose systemic subcutaneous IL-2 (10% of IL-2 dose) and systemic subcutaneous interferon alpha (IFN alpha). The effectiveness of this treatment is remarkable. No pulmonary metastases progressed during treatment. One complete response, 8 partial responses, and 6 cases of stable disease were achieved in the lungs of the 15 patients. In addition, 3 of 7 patients had partial responses and 1 of 7 had stabilization of non-pulmonary metastases. Overall response according to WHO criteria was 1 complete response, 6 partial responses, 2 mixed responses, 5 stable diseases and 1 progressive disease. Toxicity was low. Only WHO grade I toxicity occurred, except for a single grade II event (bronchospasm). This allowed long-term ambulatory treatment (1-23 months) inclusion of high-risk patients, and inclusion of patients with advanced disease. The expected mean survival of patients was 9.9 months, the actual mean survival is now 19.1 months, and 11 of 15 patients are still alive. Quality of life during treatment was good. Inhalation of IL-2 serves as a clinical model for high effectiveness and low toxicity of long-term local IL-2 application. We conclude that mainly local treatment might be the key to successful nontoxic use of IL-2 in cancer patients.
Publication Types: Clinical Trial
Huland E, Heinzer H, Mir TS, Huland H. (1997)
Inhaled interleukin-2 therapy in pulmonary metastatic renal cell carcinoma: six years of experience.
Cancer J Sci Am. 3 Suppl 1:S98-105.
PURPOSE: Patients with advanced metastatic renal cell carcinoma often cannot or do not want to tolerate high-dose systemic interleukin-2 (IL-2) therapy and the toxicity associated with it. To reduce toxicity and still maintain or even increase effectiveness, we developed a method to deliver IL-2 locally for the treatment of pulmonary and mediastinal metastases in metastatic renal cell carcinoma patients. PATIENTS AND METHODS: We report here 6 years of experience treating 116 metastatic renal cell carcinoma patients who had pulmonary or mediastinal metastases with inhaled IL-2. We have utilized three different IL-2 preparations (natural human IL-2 purified from the supernatants of mitogen-activated peripheral blood lymphocytes, glycosylated recombinant IL-2 produced by Chinese hamster ovary cells, and non-glycosylated recombinant IL-2 produced by bacteria). All protocols used high-dose inhalation of IL-2, either exclusively (11%), with coadministration of low-dose systemic IL-2 (33%), or with coadministration of low-dose systemic IL-2 and interferon-alpha (56%). RESULTS: Maximal toxicity per total treatment time was mild (median treatment time, 7.2 months); there was a low incidence (16%) of World Health Organization grade 3 toxicity. Toxicity associated with exclusive inhalation of IL-2 was local and consisted mainly of cough. Thus, patients who could not tolerate high-dose systemic IL-2 were able to tolerate inhalation IL-2 therapy. Progressive pulmonary metastases responded in 15% of patients for a median of 15.5 months (range, 4.1-33 months) and were stabilized in 55% of patients for a median of 6.6 months (range, 3-51.7 months). The overall response rate was 16%; disease was stabilized in 49% of patients and disease progressed in 35% of patients. The overall median response duration was 9.6 months. Median survival was 11.8 months (range, 1.7-68.8 months); expected survival according to risk analysis was 5.3 months. CONCLUSIONS: Inhalation of IL-2 is a nontoxic and effective treatment for patients with progressive pulmonary and mediastinal metastases. Inhaled IL-2 effectively prevented progress of pulmonary metastases in 70% of patients. Furthermore, patients could be treated as outpatients and remain employed. Local administration of IL-2 increases therapeutic effectiveness with little or no toxicity.
Publication Types: Clinical Trial, Controlled Clinical Trial
Huland E, Heinzer H. (2003)
Renal cell carcinoma: novel treatments for advanced disease.
Curr Opin Urol. 13(6):451-6.
PURPOSE OF REVIEW: IL-2 or IFN-alpha induce remissions and prolong life in patients carefully selected for a possibly toxic treatment. However, there is a need for better-tolerated and more effective therapies, especially in patients with co-morbidities and those resistant to systemic immunotherapy. Recent achievements in the treatment of advanced renal cell carcinoma highlight potentially significant improvements. RECENT FINDINGS: Cytoreductive surgery or radiation of metastases seems beneficial in well-selected patients, especially as immunotherapy is available. Immune cells within the tumour correlate with response and survival, indicating the importance of local immune modulation. Such modulation has allowed the introduction of well-tolerated treatments such as the inhalation of IL-2 to control lung metastases, which results in a significant survival benefit for high-risk patients, as suggested by a recent outcome study in 200 patients. Antibody-based tumour targeting against cG250, specifically expressed on renal cell carcinoma, seems to stabilize progressive metastatic disease and does not induce toxicity. Vaccination strategies are also well tolerated, but have not yet shown convincing results in advanced disease. Other approaches have not fulfilled expectations. Thalidomide has significant neurotoxicity and its efficacy was not confirmed in recent studies. Stem cell transplantation has significant toxicity, and cannot yet be recommended, but may have future potential. SUMMARY: Cytokine-based immunotherapy can now be considered standard in the treatment of metastatic renal cell carcinoma. There is good evidence that additional local procedures such as surgery, radiation or the inhalation of IL-2 improve response and survival in metastatic disease with moderate toxicity, resulting in a significant improvement for patients suitable for these approaches.
Huland E, Burger A, Fleischer J, Fornara P, Hatzmann E, Heidenreich A, Heinzer H, Heynemann H, Hoffmann L, Hofmann R, Huland H, Kampfer I, Kindler M, Kirchner H, Mehlhorn G, Moniak TH, Rebmann U, Roigas J, Schneider TH, Schnorr D, Schmitz HJ, Wenisch R, Varga Z, Vinke J. (2003)
Efficacy and safety of inhaled recombinant interleukin-2 in high-risk renal cell cancer patients compared with systemic interleukin-2: an outcome study.
Folia Biol (Praha).49(5):183-90.
Systemic IL-2 is an effective treatment for low to intermediate risk mRCC patients, its efficacy is marginal in high-risk cases. Therefore, other treatment approaches are required for this population. Ninety-four high-risk patients with RCC and pulmonary metastases were treated with inhaled plus concomitant low-dose subcutaneous rhIL-2. Clinical response, survival and safety were compared with those from IL-2 given systemically at the registered dose and schedule in 103 comparable historical controls. In the rhIL-2 INH group, treatment consisted of 6.5 MIU rhIL-2 nebulized 5x/day and 3.3 MIU rhIL-2 SC once daily. The rhIL-2 SYS group received treatment which consisted of intravenous infusion of 18.0 MIU/m2/day rhIL-2 or SC injection of 3.6-18.0 MIU rhIL-2. Some patients in both groups also received IFNalpha. Mean treatment durations were 43 weeks rhIL-2 INH and 15 weeks rhIL-2 SYS. Significantly longer overall survival and progression-free survival durations were observed in the rhIL-2 INH group. The probability of survival at 5 years was 21% for the rhIL-2 INH group. No patients survived 5 years in the rhIL-2 SYS group. A multivariate analysis of overall survival adjusting for differences in baseline characteristics between the two treatment groups resulted in a risk ratio of 0.43 (95% CI 0.30-0.63; P < 0.0001). The data suggested an association between the response (SD or better) and survival, especially in the rhIL-2 INH group. The inhalation regimen was well tolerated. This outcome study suggests that administration of rhIL-2 by inhalation is efficacious and safe in high-risk mRCC patients with pulmonary metastases, who have no other treatment option available.
Publication Types: Clinical Trial
Huland E, Heinzer H. (2004)
Renal cell carcinoma - innovative medical treatments.
Curr Opin Urol. 14:239-244.
PURPOSE OF REVIEW: This review highlights recent innovations of medical treatments in advanced renal cell carcinoma. Because of significant toxicity many patients do not benefit from standard systemic cytokine therapy using interleukin-2, interferon-alpha, or both. Their median progression-free survival is about 2 months and their overall survival is poor. Here we report improvements in patient outcome, focusing on overall survival, progression-free survival and quality of life to meet their needs. RECENT FINDINGS: Rating treatment results requires analysis of patient selection and risk stratification. In well-selected patients systemic interleukin-2 therapy as well as combinations of cytokines (interleukin-2 and interferon-alpha) improve outcome, induce long-term survival and, in a minority of patients, even produce long-lasting complete remissions. Within the group of patients who are not candidates for systemic cytokines, interleukin-2 for pulmonary metastases given locally by inhalation achieves long-term progression-free survival and long-term overall survival similar to high-dose systemic interleukin-2 but without the toxicity associated with it. In patients at high risk for metastatic disease progression-free survival is prolonged by autologous tumor vaccine as reported in a recent randomized study. However, in established metastatic disease no clear benefit has been demonstrated by any of the many recent vaccine protocols. Chemotherapeutic agents and thalidomide generally fail to improve patient outcome but produce significant and sometimes irreversible toxicity. SUMMARY: Cytokine-based immunotherapy can be considered standard in the treatment of metastatic renal cell carcinoma today. Long-term survival and sometimes even cure seems possible in patients using systemic and local (inhaled) applications of interleukin-2 alone or in combination with interferon-alpha. Noncytokine treatment approaches are scientifically interesting but still disappointing from a patient's perspective.
Huland E, Heinzer H, Jorres RA, Loppow D, Huland H. (2004)
Therapeutic approaches in metastatic renal cell carcinoma: local immunotherapy.
Urologe A. (Online First)
Since 1990, aerosol interleukin (IL)-2 has been used to treat pulmonary metastatic renal cell carcinoma (pmRCC). Inhalation therapy deposits a drug into the airways to achieve a high, local, clinical effect while avoiding serious systemic side effects. We report three studies to describe safety and efficacy of aerosol IL-2 in patients with pmRCC. In a multicenter study, 24 patients received exclusive inhalation (study I) of natural IL-2 (three dose levels, 48 weeks) and response and toxicity were evaluated. The survival of high-risk patients (study II) with mainly inhaled IL-2 ( n=94) was compared with that of patients receiving systemic IL-2 ( n=103). In ten patients we analyzed in detail lung function and markers of airway inflammation before and during inhalational IL-2 therapy (study III). Study I: The response of exclusive inhalation was 33.3% at 3 months and 16.7% at 6 months. Treatment was well tolerated, cough being the most frequent adverse event. Study II: The probabilities of survival at 5 years were 21% for the inhalational group and 0% for the systemic group. Study III: Inhaled IL-2 induced a moderate decline of forced expiratory volume (FEV), while exhaled nitric oxide (NO) and sputum eosinophils rose accompanied by moderate cough and dyspnea. In conclusion, inhalational IL-2 combines good efficacy and improves tolerability. This is especially important for patients who are not able to benefit from systemic IL-2 therapy. Whether the local eosinophilic response additionally supports the antitumor effect remains a challenging question.
